Emek Blair, PhD, and Alan Miller, ND
Abstract
The tri-peptide glutathione (cysteine-glutamine-glutamic acid) is one of the most important intracellular antioxidants in the body. It is assembled in the body from these individual amino acids. When the production of glutathione is faulty or overwhelmed by demand, oral supplementation of glutathione is a rational step for restoring the redox balance in the body. However, studies of oral glutathione supplementation have demonstrated poor absorption (as low as 0%) and uneven results.
Delivering supplemental glutathione via liposomal technology is the logical next step, as liposomes not only significantly increase the absorption of poorly absorbed substances, they also protect the nutrient from degradation by stomach acids and enzymes.
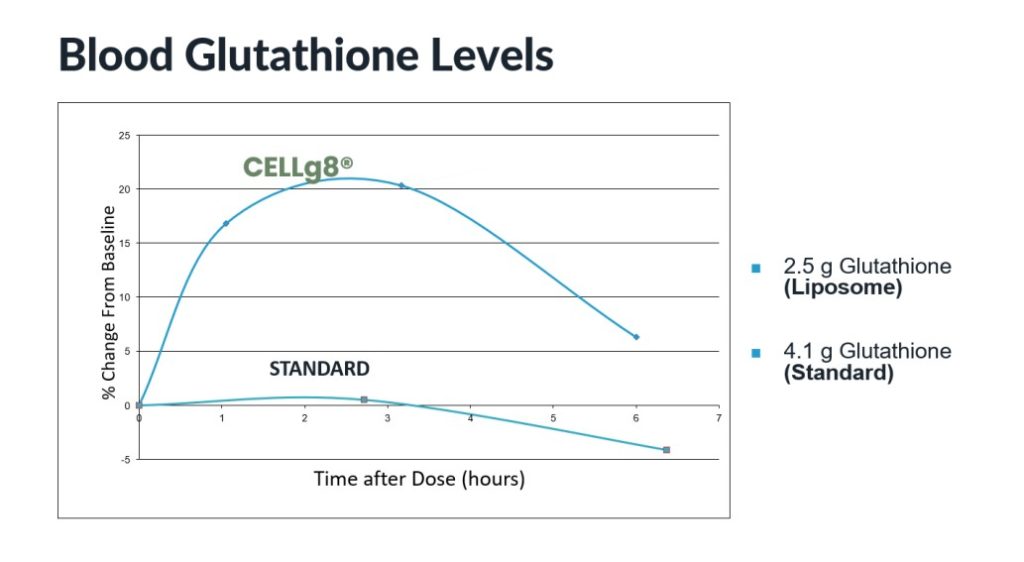
A clinical study was undertaken to assess the effect of liposomal glutathione on blood levels and detoxification processes. Seven individuals ingested a standard glutathione powder, then after a one-week washout took a liposomal glutathione liquid (CELLg8®). Peak blood glutathione was compared, with the liposomal preparation resulting in 20-times higher blood levels on average.
In another small, open-label study, seven healthy individuals took 750 mg of liposomal glutathione liquid (CELLg8®) twice daily for 30 days. Blood mercury, creatinine, and bilirubin were assessed at baseline and on day 30. Bilirubin, a marker of liver health, was significantly lower in three of the four participants, while bilirubin in the fourth individual stayed the same. Creatinine, a marker of kidney function, was also improved in three of four people. Blood mercury levels decreased an average of 39% over 30 days of supplementation.
These studies show an enhanced absorption profile of liposomal glutathione. The non-liposomal powder did not change the blood level, while supplementing with the CELLg8® glutathione liquid resulted in a blood level that was 22% higher than baseline. In addition, liposomal glutathione was safe and caused a clinically significant 39% decrease in blood mercury with 30 days of supplementation.
Introduction
The true benefits of vitamins and various dietary supplements have been discounted in the past due to low absorption and an inability to obtain a clinical dose in the bloodstream. Even the mainstay of any vitamin regimen, vitamin C, becomes less bioavailable with increased doses, especially above 250 mg.1 For example, taking either 1 or 2 grams of powdered vitamin C will yield similar blood levels, as the majority of vitamin C will pass through the body.
As in most cases, nature has already developed the solution to this problem; specifically, liposomes are used to deliver life-sustaining nutrition via breast milk.2 This liposomal delivery method can now be used to enhance absorption of materials that would either be rejected by the digestive system (e.g. vitamin C at doses over 250 mg) or destroyed by the digestive system (e.g. glutathione).
Orally administered liposomal technology has emerged as a promising remedy to low nutritional bioavailability.2 Liposomes made of synthetic materials are currently used by pharmaceutical companies for improved drug delivery.3 However, cutting edge supplement companies are using a natural methodology to deliver a variety of low-bioavailability materials that include vitamins (e.g. C, D, and B complexes), minerals such as magnesium, natural products such as curcumin and resveratrol, important cellular detoxifier and radical scavengers such as glutathione (GSH),4 and even fatty acids such as omega-3 DHA.
With each of these materials, liposomes help to overcome each nutrient’s various hurdles to absorption. Furthermore, when liposomes are made of high-quality ingredients such as phosphatidylcholine, the liposome itself provides nutritional support for the liver, brain, mitochondria, eyes, and most essential cells in the human body.
What Are Liposomes?
Liposomes can be described as submicroscopic bubbles composed of lipids, phosphatidylcholine specifically. These “bubbles” can be loaded with various materials that are delivered at high levels into the bloodstream instead of simply passing through the digestive tract.
The particle size and distribution of a liposomal system has been studied using scanning electron microscopy and nano-particle tracking analysis, which verified both a uniform and tightly packed size distribution (95% of liposomes are 50-420 nm). Furthermore, the liposomes studied here remained stable for a period of eighteen months at room temperature with respect to particle size, distribution, and homogeneity. This system is representative of an ideal sample, as the liposome size is large enough to carry a payload while remaining small enough to provide efficient absorption (less than 450 nm).
Liposomes work by initially protecting the payload (e.g. vitamin C, glutathione) from the harsh acidic environment of the stomach. Once past the stomach, the payload is delivered where needed by absorption through one of the many mechanisms available to liposomes.5 This higher absorption of nutrients or botanicals allows their full benefit to be realized. An added advantage of liposomes is increased circulation time of the payload, which ensures that each part of the body receives the nutrition it requires.
Qualities of a Natural and Effective Liposome System
- Being fluid with low viscosity and can easily be poured out.
- Opaque, as this is a colloid.
- The main ingredient must be water.
- The ingredients should be natural rather than hydrogenated or made with synthetic chemicals or a high concentration of alcohol.
Observed Effects of Liposomal Glutathione
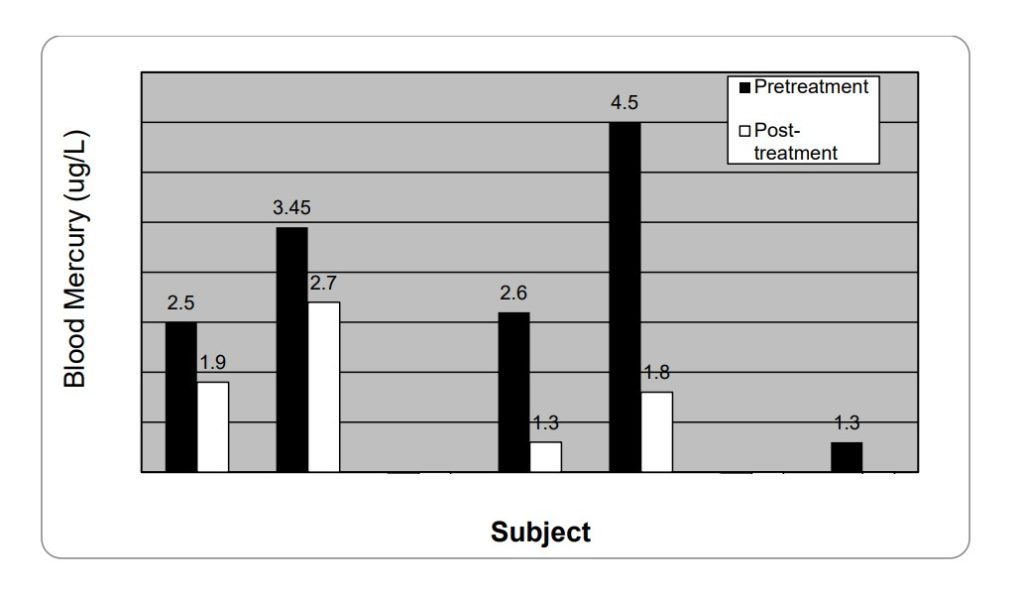
after (gray) 28 days of liposomal glutathione supplementation.
Subject 2’s day 28 blood Hg levels are below limit of quantification
(limit of quantification is 1 µg/L Hg) while both subject 3 and 6 had
below detectable levels for both pre and post treatment.
Data courtesy of Energy Medicine Research Institute, Boulder CO.
Liposomal glutathione bolsters mercury detoxification: Day-to-day exposure to toxins and toxicants such as mercury is exacerbated by continuous exposure due to power production using coal, use of mercury in energy efficient light bulbs,6 amalgam dental fillings,7 diet (e.g. fish), and inoculations.8 Traditionally, glutathione precursors such as N-acetylcysteine (the rate-limiting substrate) have been utilized in order to supply the body with the raw materials needed to increase glutathione.
As direct blood measurement of glutathione is currently unreliable, one clinic has been tracking mercury expulsion from the blood using liposomal glutathione. As a tangential biomarker, mercury, bilirubin, and creatinine were used to track the effect of twice daily doses of 750 mg glutathione and 600 mg phosphatidylcholine. Seven clients were tracked for four weeks (see Figure 1).
The initial baseline, before liposomal glutathione supplementation, was obtained from an average of blood mercury levels over two weeks (three tests at week -2, -1, and 0 averaged with a difference of no more than 0.2 µg/L). Most published data have shown that reducing blood mercury levels requires at least 8 to 10 weeks of glutathione or glutathione precursor; however, supplementation in this study showed that blood mercury levels were reduced within one week by at least 8%. when looking at the four subjects that were tracked weekly. All blood tests and phlebotomy were performed at LabCorp in Boulder, Colorado.
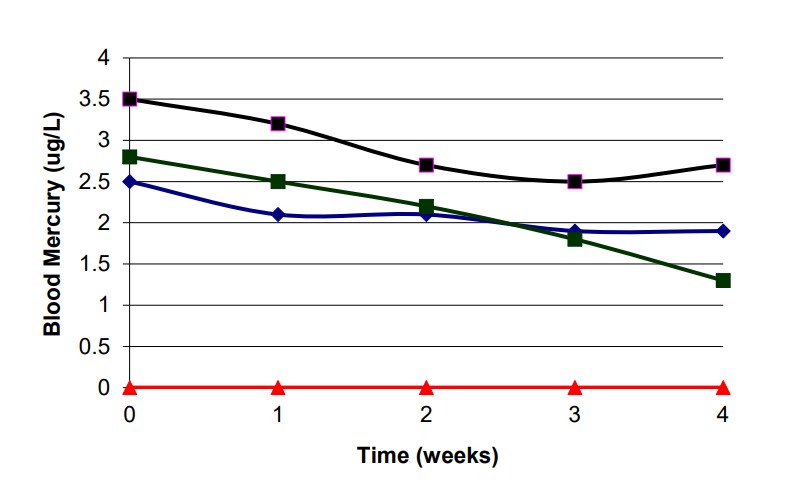
After 28 days of treatment, all five subjects showed dramatic blood Hg level reduction with a 19% – 60% improvement (Figure 1), while two subjects had pre- and post- blood mercury levels that were below detectable limits. This shows a bolstering of stage II and III of the human detoxification system.
As glutathione is critical for proper organ function, increasing glutathione levels in these subjects also improved their liver function, as their blood bilirubin levels were lowered, and improved renal activity, as their blood creatinine levels were lowered.9,10
It is interesting to note that while subject six did not have a change in blood mercury levels, this subject still saw bilirubin and creatinine improvement.
Discussion
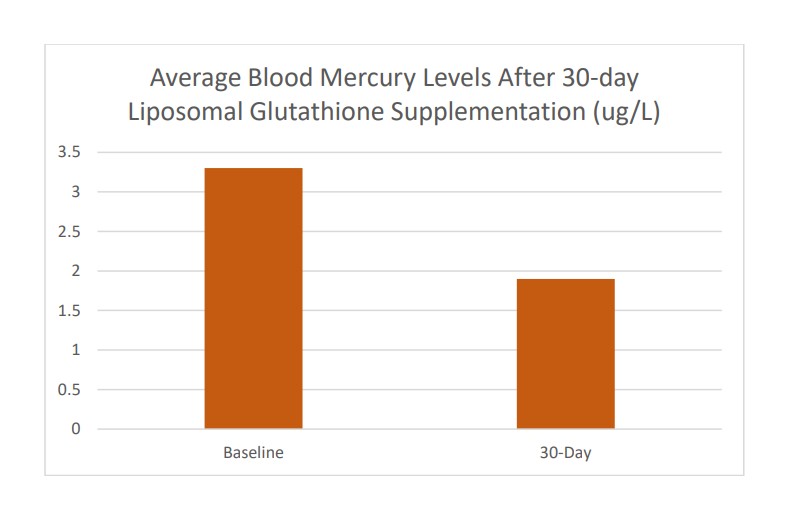
As research continues and liposomal supplements are reaching widespread acceptance, the benefit of oral liposomal supplementation is becoming clearer. These studies show an enhanced absorption profile of liposomal glutathione, with blood levels more than 20 times greater than a standard glutathione powder. The powder did not change the blood level, while supplementing with the CELLg8® glutathione liquid resulted in a blood level that was 22% higher than baseline. In addition, liposomal glutathione was safe and caused a clinically significant 39% decrease in blood mercury with 30 days of supplementation.
While this article only touches on the detoxification support provided by oral liposomal glutathione, the potential applications are vast. We already know that glutathione is directly linked to length and quality of life. Overcoming absorption issues with proper liposomal supplementation will lead to a healthier population with more personalized dietary supplementation.
Published March 11, 2023
References
- Groff, J.L., Gropper S.S., and Hunt S.M. The Water Soluble Vitamins. In: Advanced Nutrition and Human Metabolism. Minneapolis: West Publishing Company, 1995, p. 222-237
- Keller BC. Liposomes in nutrition. Trends Food Sci & Tech. 2001; 12: 25-31.
- Liposomal amphotericin B (Gilead Sciences), Liposomal doxorubicin (Zeneus), Liposomal vaccines (Berna biotech)
- Wotschi A, Reddy S, Stofer B, Lauterburg BH. The Systemic Availability of Oral Glutathione. Eur J Clin Pharm. 1992; 43:667-669.
- Torchilin VP, Recent Advances with Liposomes as Pharmaceutical Carriers. Nature Reviews, Drug Discovery 2003; 4: 145-160.
- Clakson TW, Vyas JB, Ballatori N. Mechanisms of Mercury Disposition in the Body. Am J Indus Med. 2007; 50:757-764.
- Molin M, Schutz A, Skerfving S, Sallsten G. Mobilized mercury in subjects with varying exposure to elemental mercury vapour Int. Arch. Occup. Env. Health. 1991; 63: 187-192.
- James SJ, Slikker W III, Melnyk S, New E, Pogribna M, Jernigan S. Thimerosal neurotoxicity in associated with glutathione depletion: Protection with glutathione precursors. NeuroToxicology. 2005; 26: 1-8.
- Ross EA, Koo LC, Moberly J.B. Low whole blood and erythrocyte levels of glutathione in hemodialysis and peritoneal dialysis. Am. J. Kidney Dis. 1997; 30: 489-494.
- Santangelo F, Witko-Sarsat V, Drueke T. Descamps-Latscha B. Restoring glutathione as a therapeutic strategy in chronic kidney disease. Nephrol. Dial. Transp. 2004; 19: 1951-1955.
Conflict of Interest Statement
Dr. Emek Blair owns CELLg8®, a liposomal company that manufactures dietary supplements.
Dr. Alan Miller is employed at CELLg8®
Published March 11, 2023

